The next set of ‘ambitious but achievable’ milestones for the pig sector on its journey towards more responsible use of antibiotics will include a further 20% reduction in usage, under new targets currently being developed.
This will be alongside a range of other initiatives to further build on the positive change driven by the first the two rounds of targets.
Following the influential O’Neill report on antimicrobial resistance in 2016, Responsible Use of Medicines in Agriculture (RUMA) established a targets task force to work with species sectors to agree targets around responsible antibiotic use.
In the pig sector, this has been done through the Pig Health and Welfare Council’s (PHWC) Antimicrobial Usage sub-group.
The process of developing the third round of pig sector antibiotic targets is now entering the final stages. But, according to Grace Webster, a vet with GW Pig Consultants in Scotland, the PHWC sub-group still welcomes comments and suggestions to further refine the proposal or raise concerns about its implementation at farm level.
RUMA Targets Task Force rounds |
||
| TTF | TTF2 | TTF3 |
| 62.4% reduction in use to 99mg/PCU | 30% reduction in use | 20% reduction in use |
| Reduced use of HP-CIAs | Maintain existing minimal use of HP-CIAs | Maintain existing minimal use of HP-CIAs |
| Programme to support PHUs (top 5% of each category of holding) | PHU status expanded to the top 10% of each category | |
| Monitor effect of reduced antibiotic on pig health using PHS, WPS & Pig Regen | Develop alert system for health investigation based on CCIR trend data | |
| Identify best practice weaner management |
Use of quantitative biosecurity assessment to allow monitoring of progress and benchmarking | |
| Transition away from in-feed medication |
Continue the transition away from in-feed medication | |
| Increase opportunities for medicines training | ||
Recent progress
Despite the various challenges experienced between 2020 and 2024, including the pig backlog crisis, escalating input costs and the loss of zinc oxide, the pig sector has reduced antibiotic usage by a further 18% during TTF2, the second round of targets.
With this in mind and with guidance from the Veterinary Medicines Directorate (VMD) that targets should be specific, measurable, achievable, relevant and time-bound (SMART), but also ambitious, it is proposed that the numerical target for TTF3 will be a further 20% reduction in antibiotic use.
“As part of this numerical target, we aim to maintain use of highest-priority critically important antibiotics [HP-CIAs] at their current extremely low level,” Dr Webster said.
Currently, usage is expressed as mg/ population correction unit (PCU), to be in line with terminology used in Europe, but European Sales and Use of Antibiotics in Veterinary Medicine (ESUAvet) has amended its metric to present data in mg/kg.
“This metric will give a figure that is lower for the same quantity of antibiotic used,” Dr Webster explained. “If the UK wants to continue to benchmark and make direct comparisons, we will need to change our metrics. Otherwise, our usage would appear to be higher than other countries.”
It is proposed that eMB reports will initially provide usage in both mg/PCU and mg/kg to ensure this is clear.
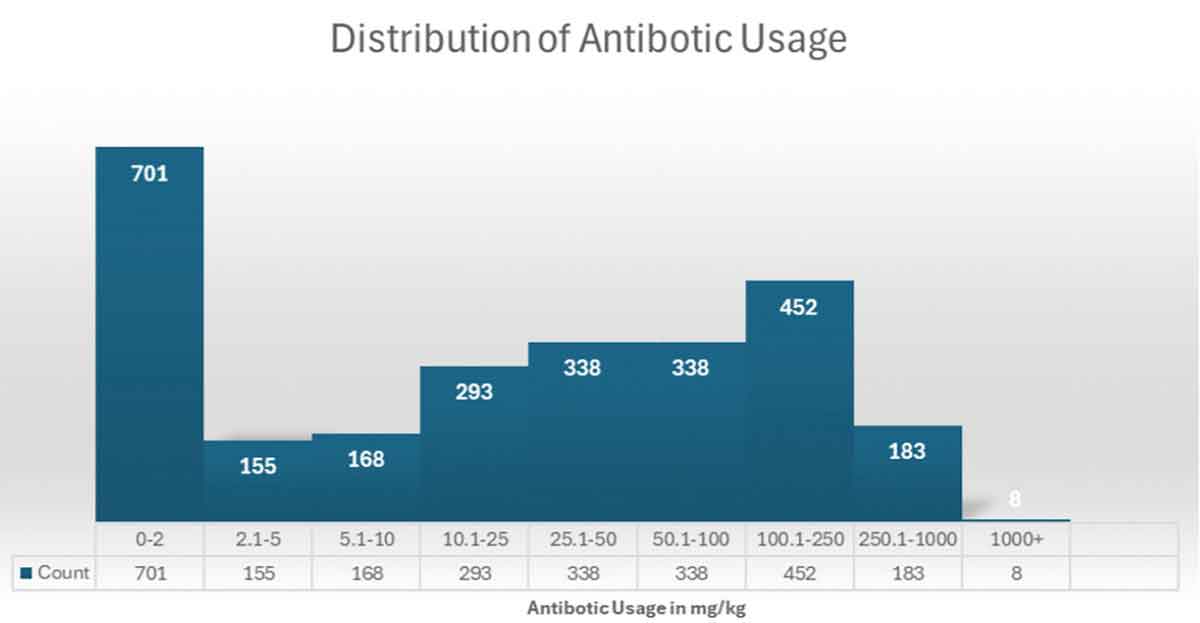
Distribution of antibiotic usage |
||
| Category | Definition | Action required |
| Red | Top 10% of use | Antibiotic reduction plan mandatory and recommendation that a numerical biosecurity audit is carried out |
| Amber | Top 10-20% of use | Advised to develop an antibiotic reduction plan – this becomes mandatory if the farm remains in the -amber category for four quarters |
| Green | Below top 20% of use | No specific action required |
Persistently high users
In TTF2, the persistently high user (PHU) was introduced and defined as the top 5% of use in each holding category – such as weaner breeder, farrow to finish, weaner and finisher – over a rolling 12-month period.
With support from Red Tractor and QMS, PHUs are required to develop an antibiotic reduction plan (ARP) with their vet, which should have timescales for implementation and be reviewed quarterly.
Only 35 units have remained as PHUs for eight quarters or more since this started, suggesting that this is an effective approach. In addition, nearly 60% of producers are using less than 50mg/PCU.
In order to build on this approach, the group agreed that it was important to expand the definition of the PHU to the top 10% of each eMB holding category, but to introduce a baseline of 50mg/PCU, below which PHU status will not be triggered.
“This will ensure that units already demonstrating good antibiotic stewardship are not asked to remove necessary antibiotics,” Dr Webster added.
Under this approach, the plan is to introduce a traffic light system (see “Traffic light system”, right) identifying PHUs and those at risk of entering the category.
“PHU status is intended to support and encourage changes in antibiotic stewardship,” Dr Webster said.
“The AMU subgroup is working on an initiative with partners to provide additional support to PHU producers to identify the factors that have led to higher use on their farms, as well as the barriers to reducing usage. The aim would be to produce interventions for PHU producers to help overcome those barriers, which would also be made available in a form that can be used across the sector.
“This would be a piece of participatory action research, with a view to commencement in 2026, if funding is successfully secured.”
Transition to in-water administration
The majority – just – of the antibiotics used in pigs are still administered in feed, but over the course of TTF and TTF2, there has been a gradual shift towards the more targeted water and injectable routes.
“While administering antibiotics in feed can be the most appropriate and responsible choice, it is also recognised that water-soluble medications can be used in a more targeted way and usually require a shorter treatment period,” Dr Webster added.
A lack of suitable water delivery infrastructure is a barrier to change, but industry representatives plan to engage with Defra to get support under the Farm Equipment Technology Fund to help address this.
Antibiotic administration
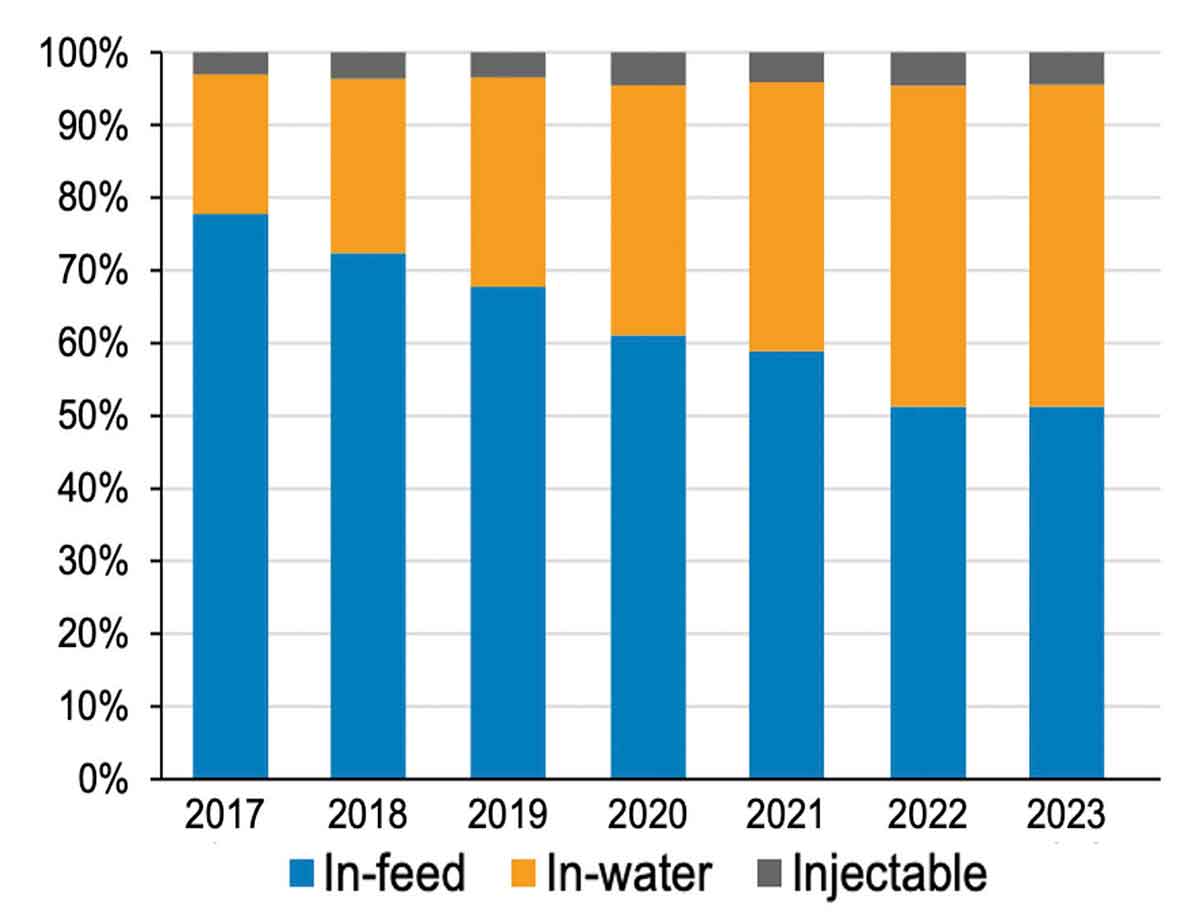
Source: VARSS Report 2023, Defra
Abattoir condemnation data
Use of abattoir health data using AHDB’s Pig Health Scheme (PHS), Wholesome Pigs Scotland and Pig Regen in Northern Ireland was a TTF2 target. However, following the withdrawal of the PHS in December 2021, the pig sector is now looking to other abattoir data sets that currently exist to continue to fulfil this important target for producers in England.
The Collection and Communication of Inspection Results (CCIR) system is used by the Food Standards Agency to record and communicate carcase and offal condemnations. Although it does not directly equate to PHS data, it could be used as a tool to highlight individual farms that would benefit from further investigation from their vet.
“A pilot scheme is in development, using data from a small number of larger food business operators,” Dr Webster said. “It aims to analyse CCIR data to highlight trends in condemnations over time. On an anonymous basis, producers will be able to use the data for tracking trends and benchmarking purposes.”
It is hoped that the pilot scheme will be in place by the end of 2026 and could be developed further beyond the end of TTF3. Producers will be able to access AHDB’s virtual abattoir tool, so they can access images of condemnations and disease lesions to give a better understanding of their herd’s health, welfare and food safety status.
Biosecurity
“It is widely acknowledged that improvements in biosecurity lead to better management of animal health, which, in turn, can reduce antibiotic usage, and lead to better productivity and profitability,” Dr Webster added.
“The pig sector continues to be challenged with significant disease events – for example, post-weaning diarrhoea following the withdrawal of zinc oxide in weaner diets, and also swine dysentery.
“Since treatment and medical elimination of swine dysentery has repeatedly resulted in high antibiotic use within the sector, this disease will be a key focus of this target.”
AHDB has already funded 50 Biocheck user accounts to allow veterinary practices to access the Biocheck biosecurity scoring tool (https://biocheckgent.com/en). This will give vets a tool to evaluate biosecurity on their clients’ farms and generate actions to make improvements.
The Biocheck tool is being used as part of the Scottish PRRSv project, so there is potential to link up the data sets to present a more GB-wide measure of biosecurity practices.
“The sub-group has already discussed with Red Tractor the possible inclusion of a requirement to carry out biosecurity audits in its next revision of the pig standards to encourage completion of audits across a wide number of producers,” Dr Webster said.
“Biosecurity generally stimulates a degree of eye-rolling, but the subgroup aims to develop education packages for both vets and farmers, and support this with case studies that demonstrate the real benefits to farmers from effecting real behavioural change.”